Luminous Global Set to Revolutionise Breast Health Awareness in the UK: Empowering Women with Life-Saving Technology
Groundbreaking technology set to change breast health history with its UK launch
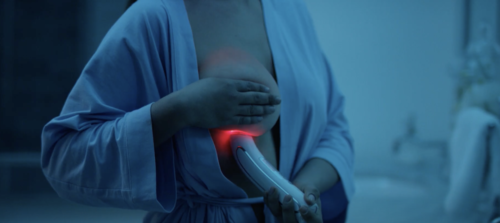
At-home and clinical breast health monitoring device: The first breast familiarity tool designed to give women and men a visual reference of their breast health.
Easy and non-invasive: Utilises CE registered Class 1 medical device transillumination patented technology with no downtime or side effects.
Published double blind IRB clinical study. Peer reviewed by Frontiers in Switzerland.
Harmless: Emits an LED red light with a harmless spectrum of 640-800 nanometers, the transilluminated breast tissue allows the observation of abnormalities represented by darker or shadowing areas.
Portable and hand-held: Small enough to be taken on holiday and used wherever you are.
Educating and empowering: Can be used by consumers or medical practitioners to track changes between clinical screenings in order for potential abnormalities to be identified.
Revolutionising breast health: Allowing early detection to save lives.
[UK, October 2024] – Luminous Pro Series breast device from Luminous Global, a revolutionary breast health monitoring device which can be used at home or in a clinical setting, is launching in the UK, empowering women to take ownership of their breast health like never before. With breast cancer being the most common cancer in the UK, affecting 1 in 7 women in their lifetime, early detection has never been more critical – and this innovative tool promises to have a game-changing impact.
Using safe, CE registered Class 1 medical device transillumination patented technology, users can visually assess their breast tissue by shining a light through the skin. This at-home and clinical device provides an easy, non-invasive method for early breast health monitoring, offering the powerful ability to track changes between clinical screenings. It is also certified for medical use meaning it can also be used in a medical setting, by doctors and nurses in hospitals and clinics.
With breast cancer accounting for approximately 15% of all cancer deaths in UK women, the importance of breast self-examinations and timely medical consultations cannot be overstated. Studies have shown that early detection significantly improves survival rates, but many women struggle with consistent self-examinations due to a lack of confidence or knowledge. Luminous Global aims to fill that gap by providing an accessible tool that can be used regularly and easily.
“This is not just a product; it's a really powerful movement toward proactive care and education. Our mission is to arm women with the resources to take charge of their breast health and of course, save lives,” says Kirsty Lawson, CEO of Luminous Global UK.
“The Luminous Pro Series Breast device represents the first line of defence for breast surveillance and is designed to be user-friendly and effective for everyone—regardless of gender, race, skin tone, breast size, or any prior chest surgeries, including augmentation, reduction, or mastectomy.
“This groundbreaking tool enhances breast self-exams, clinical exams, and supports diagnostic channels. Utilising our patented Red LED technology, the Luminous Pro Series Breast device illuminates the soft tissue of the breast, helping to establish a personalised baseline by mapping arterial blood flow. This allows for the early identification of any deviations from normal circulation, with visual darkness or shadowing potentially indicating abnormalities.”
“I believe the Luminous Pro Series Breast device would be a valuable addition to hospitals and clinics, allowing the availability to provide patients with the highest standard of care. Our shared goal is to enhance breast health monitoring and promote early detection and treatment of breast cancer on a global scale.”
Kirsty, who is a mother and community leader, shares how her own personal experience with breast abnormalities makes this a project which she is particularly passionate about.
“Last year I had a mammogram and afterwards I was notified that they had picked up an abnormality on the mammogram and that I needed to go back for further exploration.
I had an agonising four week wait until the next appointment, after which I was fortunately told that the abnormality on the scan had actually been caused by an air pocket which had shown up and was nothing to worry about. This meant that I had spent four weeks of unnecessary worry, which I think could have been significantly reduced, if not avoided, if I had had the Luminous Pro Series Breast device to check my breasts.”
The use of this device is not intended to replace the mammogram as the gold standard for breast-screening but rather to use it as an adjunct or complement tool as part of more efficient earlier detection strategies.
– ENDS
For more information please visit www.pinkluminousbreast.com/ For media enquiries, please contact Rachel Tompkins at SA Communications - rachel.tompkins@sa-comms.com, 0203 727 0726
About Pink Luminous Breast
Luminous Global is a cutting-edge health technology company committed to developing life-changing innovations that empower individuals to take control of their personal health and well-being. Launched in the US, Luminous Pro Series Breast is one of the company’s pioneering products designed to revolutionise breast health monitoring. Utilising safe, CE registered Class 1 medical device transillumination technology, Luminous Pro Series Breast device provides women with a non-invasive, easy-to-use tool for early detection of potential breast abnormalities. The device is designed to complement regular medical screenings, offering women the ability to monitor their breast tissue at home and track changes between clinical visits.
This press release was distributed by ResponseSource Press Release Wire on behalf of SA Comms Ltd in the following categories: Health, Women's Interest & Beauty, for more information visit https://pressreleasewire.responsesource.com/about.